BIOACTIVE SKIN SUBSTITUTES
Large skin damages are affected by pathogen invasion, moisture loss and inflammation at the wound site causing scarring, lifelong disfigurements or death. Despite advances in treatment, mortality due to burns greater than 70% of the body surface still remains high. Deep dermal burns suffer from loss of all integumentary structures, thereby healing slowly along with extensive scarring. Chronic wounds like pressure ulcers, venous ulcers and diabetic foot ulcers may lead to amputation in several affected patients. When the natural wound healing machinery becomes inefficient, therapeutic treatment using skin regeneration substitutes becomes imperative. An ideal skin substitute should have high porosity, swelling property, degradability, biocompatibility, optimal mechanical properties, vapor permeability and antimicrobial activity. Various skin regeneration products are currently available in the market but have certain limitations such as graft donor availability, high cost, immune rejection and risk of pathogen transmission. Moreover, these products require frequent dressing changes, thereby making wounds more susceptible to microbial infections, causing damage to neo-tissue and also increasing the cost. Thus, scientists have focused on developing biodegradable, partial or full-thickness skin substitutes from natural or synthetic biomaterials.
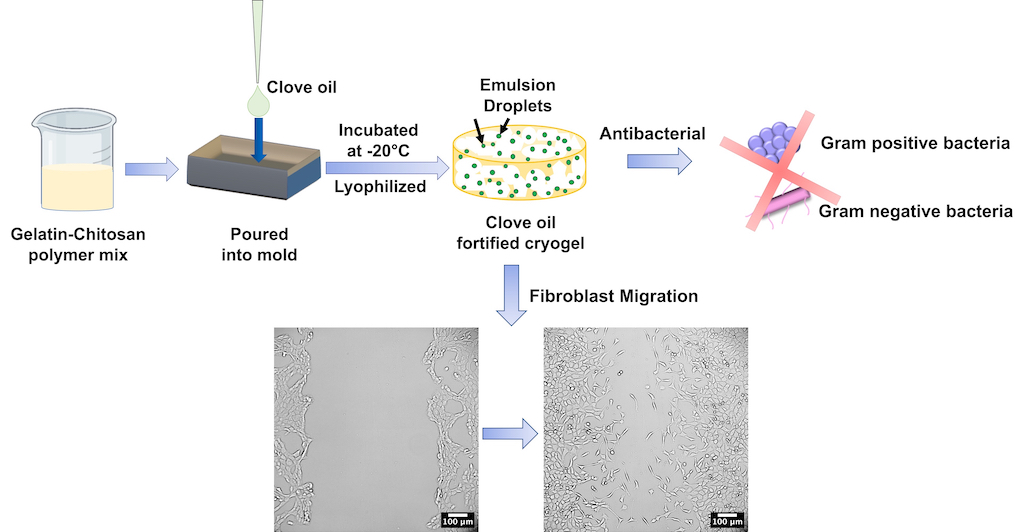
We developed an antibacterial dermal substitute by incorporating clove oil into gelatin-chitosan cryogels since infections are a leading cause of mortality and amputations among patients of burns and chronic wounds, respectively. Since extensive use of antibiotics has led to rapid spreading of drug resistance among microorganisms, plant-derived natural products, which have been used as traditional therapies for several centuries, are recently gaining popularity. They are relatively affordable and easily available in many developing countries where modern medications are expensive or unavailable. The oil-incorporated cryogels were macroporous, biodegradable, possessed mechanical properties similar to commercial skin substitutes, were cytocompatible, antibacterial and allowed long-term sustained release of oil up to at least 14 days. Additionally, clove oil aided faster closure of in vitro scratch wounds by improving migration of fibroblasts. This work presents a novel, bioactive scaffold that has the potential to be used as an alternative to commercial dermal substitutes that presently lack a bioactive component. However, a limitation of the study was absence of bioactive agents that could enhance cell proliferation or anti-inflammatory properties. In addition, clove oil emulsions were not stable for a prolonged period and tend to coalesce.
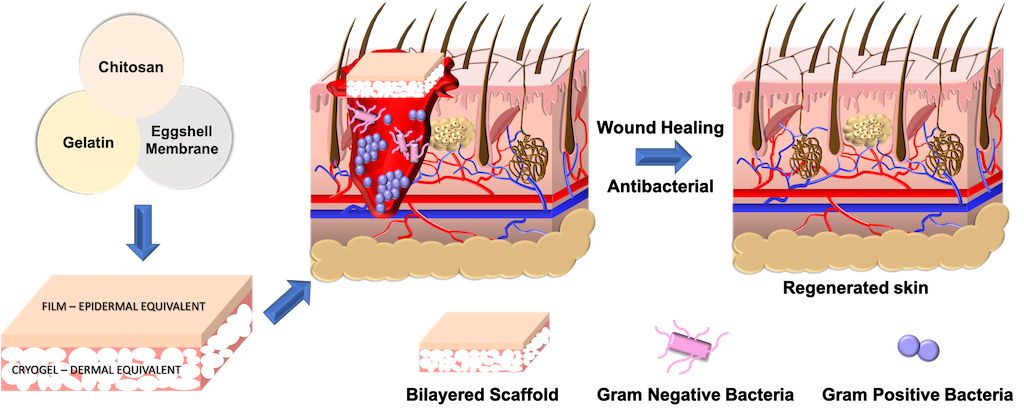
To address the aforementioned limitations, eggshell membrane powder (ESM) was used as an alternative to growth factor. It was serendipitously discovered that ESM could also promote physical crosslinking, thereby replacing glutaraldehyde, a known cytotoxic chemical crosslinker. Thus, we developed ESM-crosslinked gelatin-chitosan cryogels. The resultant macroporous cryogels with pore size range between 10-350 μm had improved flexibility, biodegradability and biocompatibility compared to glutaraldehyde-crosslinked cryogels. To recapitulate key aspects of skin physiology, we fabricated a bilayered substitute by coupling the ESM-crosslinked cryogel (dermal equivalent) to a non-porous, physically-crosslinked gelatin-chitosan film (epidermal equivalent). The epidermal layer provided the requisite barrier properties while the dermal layer facilitated cell attachment and migration for optimal wound healing. Further, chitosan conferred antibacterial property to the cryogels with almost 50% reduction in bacterial viability. Animal studies confirmed that the developed bilayered skin substitute was non-allergic, aided wound healing by improving re-epithelialization within 14 days and supported formation of skin appendages.
In a subsequent study, we used an isolated phytoconstituent to impart antioxidant and anti-inflammatory properties to the skin substitute. Rutin nanoparticles, with better aqueous solubility, were synthesized without a polymeric encapsulant and incorporated into the ESM-crosslinked gelatin-chitosan cryogels to treat prolonged inflammation, since that leads to hypertrophic scarring in large burns and delayed healing in chronic wounds. The resultant nanoparticles were 17.5 nm in size and could be stably stored at room temperature until at least a month. They were non-cytotoxic, exhibited antioxidant property by controlling reactive oxygen species generation and increasing catalase production in human macrophages and also showed anti-inflammatory property by reducing transcription of TNF-α and IFN-γ genes. Additionally, the nanoparticles could reduce α-SMA expression in fibroblasts, thereby demonstrating anti-scarring effect. In vivo studies with the bilayered skin substitute constituting this bioactive cryogel proved that it was biocompatible, aided wound healing and induced better re-epithelialization than control wounds. Renal toxicity due to rutin nanoparticles was absent. Thus, rutin nanoparticle-incorporated bilayered skin substitute is an advanced and novel alternative to commercial dermo-epidermal substitutes such as Integra, a gold-standard.
